2 Capping Overview
This section describes the different capping objectives, with a focus on the chemical isolation function of the cap, as well as general cap types and configurations. A recommended framework for cap chemical isolation design is also presented in this section.
2.1 Objectives of Capping
Capping is the process of placing one or more clean layers of sand, sediments, or other material(s) over contaminated sediments to mitigate risks to human health and the environment (ITRC 2014). Capping often includes combinations of materials, addition of amendments, and/or inclusion of synthetic materials (i.e., geotextiles, reactive mats, etc.) in the cap design to aid in the physical separation of cap layers and/or geotechnical stability, to enhance protectiveness, to maintain cap integrity, and/or to enhance its chemical isolation performance.
Cap design depends on the site-specific performance objectives, risks, and/or cap function(s). As noted in Section 1, the three primary objectives of capping include physical stabilization, chemical isolation, and protection of the benthic community (ITRC 2014). These cap functions are generally interrelated and may be combined to achieve site-specific performance objectives. This guidance assumes that a given cap’s design will inherently physically stabilize the underlying contaminated sediment, and that protection of the benthic community from underlying contamination will be achieved via the chemical isolation function. The focus of this guidance document is the chemical isolation function of the cap; however, a brief overview of each performance objective is described below to provide context.
2.1.1 Physical Stabilization
Caps must physically stabilize underlying contaminated sediment and remain resistant to erosion to prevent resuspension of contaminated sediment and subsequent transport of contaminated sediment to uncontaminated (or less contaminated) areas of a water body. In most cases, physical stabilization and erosion protection measures are functional components of a cap in areas where potential for cap disturbance is expected, such as areas with high hydrodynamic forces, areas subject to seismic events, areas with heavy navigation-related propwash (e.g., a harbor or port), areas with intermittent or prolonged exposure to air (e.g., intertidal areas, areas with drought conditions), or where high slope angles may cause failure of other cap components. In initiating any cap design process, an evaluation of the potential for erosion and an evaluation of the physical stability of sediments within the project area are critical, and if necessary, physical stabilization and erosion protection measures should be incorporated into the cap’s design to ensure the cap remains in place. Since this guidance focuses on the chemical isolation function of the cap, the specific approaches or best practices for assessing and designing for physical stability or erosion protection are not discussed explicitly. Nevertheless, this guidance recognizes that these factors are critical to long-term cap performance. The design considerations for erosion protection layers are briefly discussed in the 2005 USEPA Guidance (2005).
2.1.2 Chemical Isolation
This guidance is intended to assist users in the design, construction, and monitoring of the cap chemical isolation function. Cap designs must achieve long-term chemical isolation by inhibiting the migration of contaminants from underlying sediments, reducing risks to receptors. Achieving chemical isolation depends on site-specific factors that are discussed throughout this document. A cap design may incorporate one or more layer(s) to achieve chemical isolation through contaminant sequestration, contaminant degradation, or physical isolation of the contaminated sediment.
In addition to capping, a chemical isolation function may also be established through in situ treatment, where amendments are mixed into the underlying sediment. In situ treatment consists of placing amendments directly on the sediment surface where they mix with sediment in the biologically active zone (BAZ) and promote reduction in exposure concentrations without necessarily providing physical isolation. In situ treatment is discussed further in Chapter 4.0 of the 2014 Contaminated Sediments Remediation guidance document (ITRC 2014). Many of the principles and approaches discussed herein, particularly modeling and construction considerations, also relate to in situ treatment.
2.1.3 Protection of the Benthic Community
Protection of the benthic community or aquatic life from contaminants is a primary reason for designing and constructing a cap. In addition, habitat restoration may be identified as a capping objective. In many cases, protection of the benthic community can be achieved by designing a cap to physically stabilize the underlying contamination and provide chemical isolation; however, these design functions may not provide the best conditions for benthic or aquatic life recovery. In many aquatic systems, the benthic community represents the base of the food chain. To best protect or enhance the benthic community, information should be collected to determine the type of biological/ecological community present or desired at the site, the level/depth(s) of bioturbation that is expected to occur at the site, the thickness of the BAZ, and the physical characteristics of substrate needed to support or enhance the existing or desired benthic community. Cap design should consider this information with the objective of promoting ecosystem recovery to the extent practicable, in addition to protecting it from chemical contaminant impacts.
Although this guidance will address benthic community protection via the cap chemical isolation function, it does not discuss specific approaches or best practices for building/restoring/enhancing the function of a benthic community. In general, the thickness of the cap should be greater than the BAZ and bioturbation depths to ensure that benthic organisms in the BAZ do not come into contact with underlying contaminated sediments due to bioturbation or other mixing processes (e.g., propwash), which in turn will compromise the cap’s ability to achieve performance objectives.
2.2 Chemical Isolation Function
The cap chemical isolation performance objective, whether achieved through the use of a single cap layer or a combination of multiple cap layers, is intended to contain or limit contaminant migration and exposure to contaminants of concern (COCs) from the underlying contaminated sediments. As described in more detail in Section 3.3.1, contaminant migration through caps generally occurs via advection, diffusion, and/or dispersion of porewater; bioturbation; or ebullition. Chemical isolation may be achieved by using the thickness of a cap to create distance between and separate the underlying contamination from the overlying benthic community and surface water and/or incorporating amendments to retard the migration of contaminants through the cap. Chemical isolation can be accomplished through one or a combination of the following mechanisms; the types of cap materials would be selected based on the site-specific conditions and contaminant properties:
- physical separation, physical stabilization, or physical sequestration of contaminants
- reduction in the advective and/or diffusive flux of contaminants from sediment porewater to the overlying surface water (i.e., through the use of permeability control or adsorptive/reactive amendments)
- reduction or sequestration of contaminant flux due to gas ebullition
- degradation of the contaminants themselves
The cap design must consider the nature of contamination, potential for groundwater advection, hydrodynamics (erosional/depositional setting), anthropogenic use (e.g., navigation, commercial and recreational prop wash, anchoring/spudding), characteristics of the BAZ, and other factors.
A physical barrier alone may result in chemical isolation where contamination has limited mobility and is not expected to migrate upward through, or around, the capping layer. Use of physical barriers to achieve chemical isolation should consider the following design objectives:
- Prevent potential receptors from penetrating the cap and contacting the contaminated sediment. This form of physical barrier is typically constructed with a layer of thick or otherwise durable material (e.g., sand or stone) that prevents direct contact with the contaminated sediment.
- Inhibit the migration of contaminants through the cap. Migration of solid-phase contaminants can be limited by durable material (e.g., sand or stone) or relatively impermeable barriers (e.g., bentonite-based or geosynthetic materials).
Physical barriers should be evaluated consistent with the approach described in Section 3 to confirm they can achieve suitable chemical isolation.
Over time, caps may become recontaminated due to factors unrelated to physical integrity/stability and chemical isolation. A common cause of cap surface recontamination is deposition of contaminated sediments over the cap surface (“top-down” process). In other cases, declining cap performance may be a result of physical damage, instability, or expected or unexpected migration of contaminants from underlying sediments through the cap (“bottom-up” processes). In these cases, cap performance monitoring data can be used to ascertain whether the loss of cap integrity or other causes of declining performance are affecting remedy effectiveness, as described in Section 7.4.
2.3 General Cap Types
To accomplish the chemical isolation function, a range of cap configurations and materials can be applied depending on site conditions and performance objectives. Although many cap layers or combinations of layers can be designed to meet possible site-specific performance objectives, the three most commonly referenced cap types are unamended granular caps, low-permeability caps, and amended caps. Each is described briefly below:
- Unamended granular caps are typically constructed of sand, stone, or other natural materials (including dredged material) and are permeable in nature. Unamended granular caps are generally intended to create distance between the underlying contaminated sediment and the overlying benthic community and surface water, reducing the diffusive flux of contaminants to the BAZ and surface water and the accumulation of contaminants in the BAZ.
- Low-permeability caps are typically constructed of clay-based materials, mixtures with Portland cement, and/or geosynthetic liners. These materials are designed to provide a relatively impermeable or low-permeability barrier between contaminated sediments and the overlying benthic community and surface water, reducing the advective flux of contaminants to the BAZ and surface water.
- Amended caps may be constructed from a wide range of materials that are typically permeable but are intended to sequester contaminants and limit contaminant flux through the cap by the application of sorptive or reactive amendments. These amendments can be mixed within granular materials or can be applied as commercially available products in bulk or in mat-based forms. The type of amendment and how it is used to achieve the cap’s chemical isolation function depends on the types and concentrations of contaminants impacting the underlying sediments. Table 2-1 summarizes amendments available for use in caps. A detailed description of these amendments is included in Appendix B.
Table 2-1. Summary of amendment use for sediment capping
| Amendment | COCs Addressed | Mode of Action | Reference |
| Low-Permeability Clays | All COCs | Reduction in the advective transport through reduction in permeability | (Danny Reible et al. 2006) |
| Activated Carbon | Hydrophobic organic compounds like dioxins/furans, pesticides, PAHs (including petroleum compounds at MGP sites), VOCs, and PCBs Mercury and organometals such as methylmercury | Adsorption onto and within the activated carbon matrix | (Patmont et al. 2015) |
| Organophilic Clays | NAPL such as fuels and heavy oils (e.g., NAPL present at MGP sites) Organometals such as methylmercury Divalent heavy metals | Surface and interlamellar adsorption | (D Reible et al. 2007) |
| Zeolite | Divalent heavy metals | Surface adsorption | (Zhang et al. 2016) |
| Apatite | Divalent heavy metals | Precipitation onto phosphate minerals | (Knox et al. 2016) |
| Siderite | Divalent heavy metals | Primarily pH neutralization but contributes to adsorption and mineralization | (O’Day and Vlassopoulos 2010) |
| Bioaugmentation / Nutrient Addition | Biodegradable organic compounds Organometals such as methylmercury | Promotion / suppression of crucial biological processes that increase or reduce the formation of toxic compounds | (Matthews et al. 2013) |
| Zero-Valent Iron | Halogenated aromatic organic compounds (e.g., PCBs, chlorinated pesticides, and dioxins/furans) Divalent heavy metals | Reductive dehalogenation Reduction to mineralized/elemental metal | (Zhang et al. 2016) |
| Activated Alumina / Aluminum and Iron Oxide (Steel Slag) | Mercury Divalent heavy metals | pH precipitation and surface complexation onto the metal oxides | (Gavaskar et al. 2005; Shin and Kim 2016) |
| Manganese Oxides | Mercury or methylmercury Divalent heavy metals | Surface adsorption | (Vlassopoulos et al. 2018; Matocha, Elzinga, and Sparks 2001; Lee et al. 2011) |
| Ion Exchange Resins | Divalent heavy metals | Ion exchange/chelation | (Burgess et al. 2000) |
| Engineered Materials—ATS, Thiol–SAMMS | Mercury or methylmercury Divalent heavy metals | Surface adsorption | (Gilmour et al. 2013; Kwon et al. 2010) |
| Sulfide Minerals | Mercury or methylmercury Divalent heavy metals | Surface adsorption Sulfide mineralization/ precipitation | (Ou et al. 2020) |
| Other Carbonate Minerals | Arsenic (III), hexavalent chromium High pH control | Surface adsorption pH buffering | (Guo et al. 2011; Bibi et al. 2018) |
| Biomaterial Byproducts—Oyster Shell Powder, Chitosan | Nutrient removal (nitrogen and phosphorus) Divalent heavy metals | Surface adsorption Surface complexation | (Huh et al. 2016; Zhong, Liu, and Tang 2021; Yong et al. 2015) |
| PFAS Targeted Amendments (e.g., RemBind and FluoroSorb) | Binding PFAS compounds | Surface adsorption | (Najm et al. 2021; Stewart and McFarland 2016) |
Acronyms and Abbreviations:
COC = contaminant of concern
MGP = manufactured gas plant
NAPL = nonaqueous-phase liquid
PAH = polycyclic aromatic hydrocarbon
PCB = polychlorinated biphenyl
PFAS = polyfluorinated alkyl substances
VOC = volatile organic compound
2.4 Cap Layers and Composition
Each cap layer or group of layers should perform a specified function or functions in achieving the overall performance objectives. In some cap designs, a single cap layer may serve multiple functions and achieve more than one performance objective. For example, an unamended cap composed of sand may be used to provide a distance separating the contaminants and receptors, and the sand material may also provide habitat substrate for certain aquatic organisms. In other cases, caps may be designed with a series of layers/components, each with unique and specific performance objectives. Therefore, individual cap layers or groupings of layers are referred to as “functional layers” within an overall design.
Within this guidance document, the term “functional layer” will refer to both single layers and/or groupings of layers, based on the intended function. It is important to note that caps are designed to achieve site-specific performance objectives. Therefore, some cap designs may include only one or two of the functional layers identified below, while other designs may require multiple function layers to achieve the site-specific goals.
Caps may be monolayer or composite caps. A monolayer cap design uses a single material (e.g., sand) to achieve performance objectives. A composite cap uses different materials, each performing a separate function that, together, achieves the performance objectives. Composite caps may include natural and geosynthetic materials.
Previous guidance documents conceptualize cap layers by function (e.g., chemical isolation, operational, erosion protection, consolidation, bioturbation, etc.) while acknowledging that some cap layers may alter over time or perform multiple functions (M.R. Palermo, Clausner, et al. 1998; M. Palermo, Maynord, et al. 1998). This approach can be used to inform cap design, but most current cap designs describe cap layers using the convention presented below (listed in ascending order from the sediment upward to the water column). The cap layers discussed in this section may be used to perform multiple functions or may not be used at all for a given cap design.
2.4.1 Base Layer
A base layer may initially be placed to create an even and/or more stable base substrate on which to place the remaining cap layers. In addition, the base layer may be placed to limit mixing of the contaminated sediment with the capping media. This base layer is typically constructed using sand but may include geotextiles where the geotechnical stability of the underlying sediments is considered a significant concern for the stability of overlying capping layers. When used, this layer is expected to mix with the underlying contaminated sediment during placement and therefore is not expected to provide chemical isolation. Some caps use a base layer to serve one or more of these functions:
- limit intermixing of contaminated sediment with the CIL
- reduce the concentration of contamination in direct contact with the cap
- provide a protective layer that has capacity for controlling underlying sediment porewater that may be extruded during consolidation due to the weight of the cap material
- create added geotechnical stability to soft sediment
- create shallower slopes and improve cap constructability
- stabilize dredge residuals prior to capping in cases where dredging is performed in advance of capping
2.4.2 Chemical Isolation Layer
The CIL performs the chemical isolation function described in Section 2.2, above.
2.4.3 Filter Layer
The filter layer is typically used when there is a large difference in grain size between different cap layers and is intended to mitigate piping of the finer-grained CIL materials up, into, and through the coarser-grained erosion protection layer over time. When required, the filter layer is positioned directly beneath the erosion protection layer. It is typically constructed using medium-grained sand, gravel, or stone or geosynthetics. In some cases, multiple filter layers may be necessary (e.g., transitioning from a fine sand-based CIL to a large rip rap-based erosion protection layer).
2.4.4 Erosion Protection Layer
An erosion protection layer is intended to protect the underlying material from erosive forces that scour the surface of a cap over time. Natural erosive forces include currents, tidal forces, wind-driven waves, ice scour, etc. Anthropogenic forces include propwash, vessel-generated waves, etc. The erosion protection layer can be constructed from a range of coarse-grained natural (e.g., gravel, boulders) and manufactured materials.
2.4.5 Habitat Layer
This uppermost functional layer accommodates the benthic community and vegetation after recolonization. In some cases, the cap materials placed for chemical isolation or erosion protection provide a suitable habitat, and a distinct habitat layer is not included in the cap design. In other cases, a distinct habitat layer is incorporated into the cap design. Natural sedimentation also may contribute to the habitat layer. The decision to incorporate a distinct habitat layer should be made on a project-specific basis. Where a distinct habitat layer is placed, it should be noted that the original habitat layer thickness and composition may change over time as a result of deposition and/or erosion.
2.5 Chemical Isolation Design, Construction, and Monitoring Process
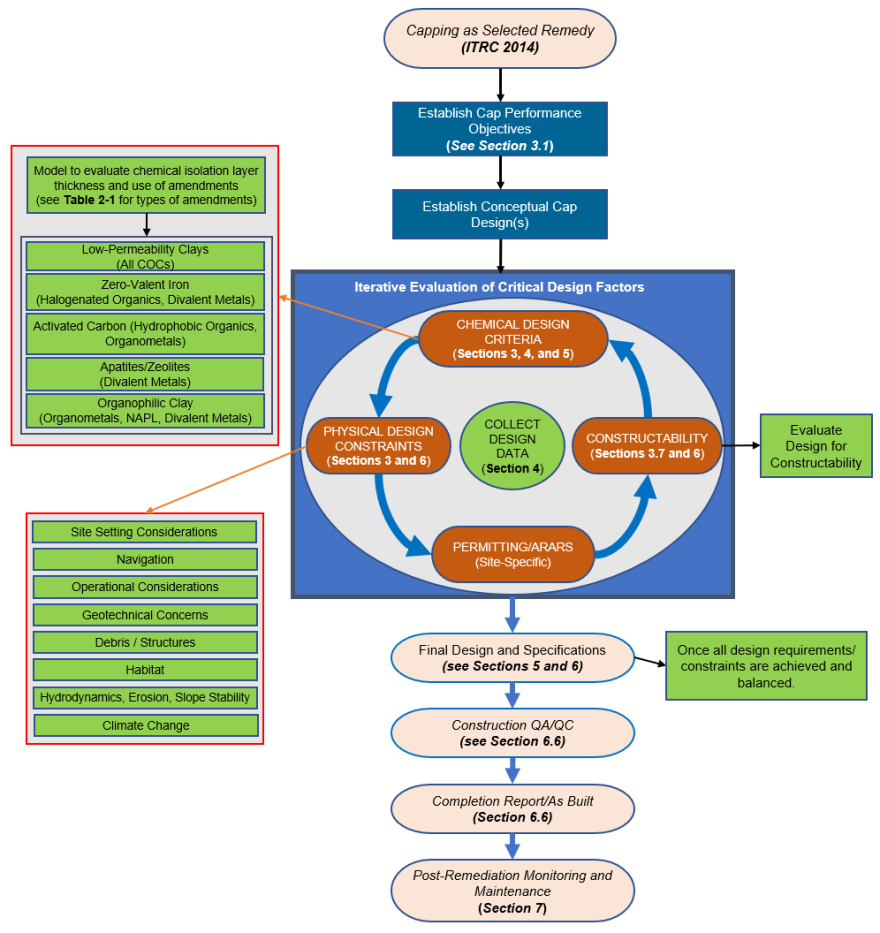
This guidance supports the use of the iterative process illustrated in Figure 2-1 to drive the cap design. Beginning with the establishment of cap performance objectives, site-specific information will drive the user toward the design that is most likely to succeed. These elements are summarized in Figure 2-1 and are discussed in detail in Sections 3 through 7.
Figure 2-1. Recommended Framework for Sediment Cap Design